Questions that inspire me:
What makes a brain “flexible”?
My interest in flexible neural coding was nurtured through early lab experiences as an undergraduate where I studied how social isolation alters the behavior of ants. With even brief social isolation, this very social creature rapidly changed how it responded to once familiar nestmates: from cooperation to aggression. Their brains hadn’t grown more neurons, but suddenly they had shifted into a new mode of action purely based on a change to their physiological state. I realized then that brains were not merely static bundles of nerves but dynamic integrators of experience.
Inspired by my time with the ants, I pursued a PhD to study how physiological state changes altered neural activity. My graduate work highlights how neuromodulation, a cellular process which alters the intrinsic properties of neurons and the synaptic strength between circuit partners, alters olfactory coding based on physiological state. Competing behavioral needs arising on a continuum (e.g. the need to eat and sleep), and these state changes are often mediated by the release of neuromodulators. As a Ph.D. student I found that neuromodulators released from different neurons anatomically converge on olfactory neurons but physiologically diverge to alter their activity in distinct ways. This physiological divergence creates unique neural signatures for each state change, allowing networks to coordinate shifts in coding along alongside overlapping behavioral needs.
How does context change neural circuit and network function?
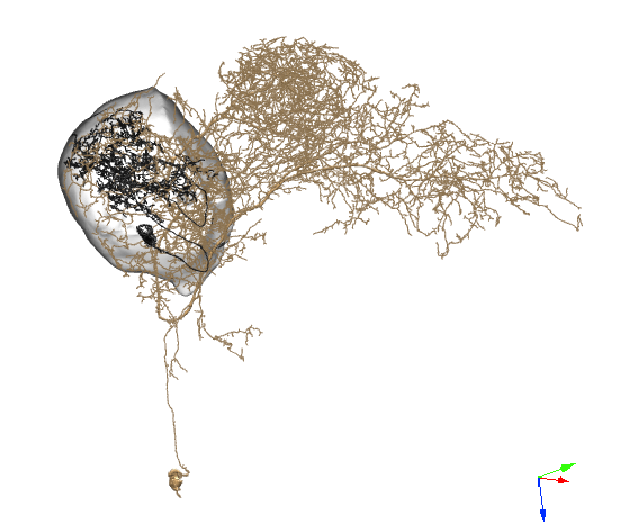
Recent advances have allowed us to create a ‘roadmap’ of all the connections between neurons in the fly brain. Leveraging this connectivity data, we can identify all the synaptic partners to and from neurons of interest and make predictions about physiological function. Enhancing this known connectivity map, we can then ask, how does context change information routing between neurons and circuits? My postdoctoral work demonstrates that odors are dynamically encoded across time, where the experience of one odor suppresses (or enhances) responses to subsequent odors. This, and my work on neuromodulation, highlight the importance of understanding circuit dynamics beyond a static snapshot of connectivity.
How I answer those questions:
-
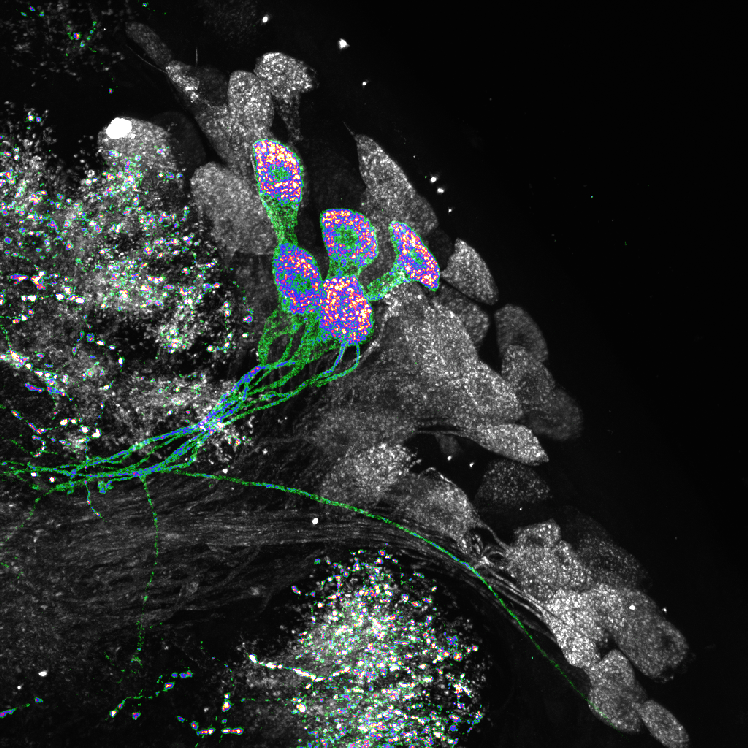
I use immunohistochemistry, neural tracing, and connectomic analysis to understand the fine-scale anatomical properties of neurons and the synaptic connections between them.
-
Using in vivo electrophysiological techniques (whole-cell patch clamp electrophysiology), and 2-photon optical imaging of voltage and calcium, I study the physiological properties of both individual neurons and populations of neurons.
-
Drosophila has an unparalleled toolbox of genetic tools to manipulate receptors, neurotransmitters/modulators, and signalling cascades with single-neuron specificity. I leverage these tools to understand the cellular and molecular basis of flexible neural processing. Using highly spatially specific optogenetic manipulation, I interrogate the physiological relationship between identified synaptic partners in vivo.
-
Drosophila may be small, but they have an impressive olfactory behavioral repetoire. Using classic fly-on-a ball behavior, and T-maze choice assays, I will assess how perturbations to specific neurons and pathways disrupt olfactory acuity, the ability of the flies to detect and respond to odors.